Countries ▼
PRE-CLINICAL STUDIES
In vitro analysis
KANJINTI® was compared against the trastuzumab reference product with respect to the primary mechanisms of action/potency and antibody-dependent cell-mediated cytotoxicity (ADCC).1,2
Biosimilarity was proven by the robust strength of the comparison data.
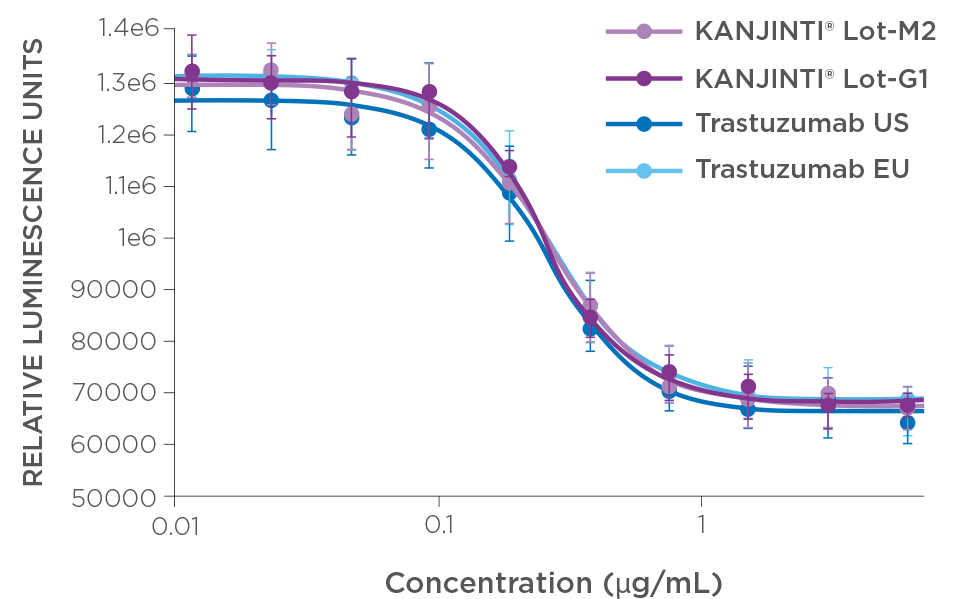
PROLIFERATION INHIBITION IN BREAST CANCER CELLS
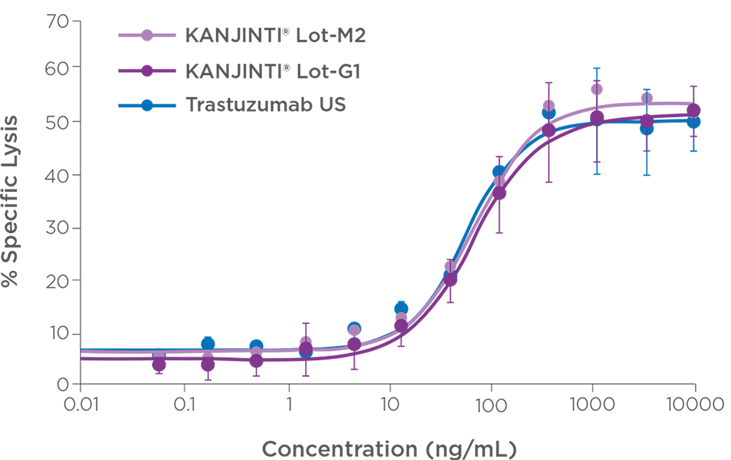
ADCC IN HER2-OVEREXPRESSING CELLS
ADCC = antibody-dependent cell-mediated cytotoxicity; PI = proliferation inhibition; RLU = relative luminescence units.
* The functional activity of KANJINTI® and EU-authorised trastuzumab reference product were compared by measuring PI and ADCC activity in HER2-overexpressing cell lines. PI activity was evaluated using BT-474 cells; ADCC was assessed using HCC2218 as target cells. Antitumour activity was compared in two mouse xenograft models: a BT-474 breast tumour model and an NCI-N87 gastric tumour model.
* The functional activity of KANJINTI® and EU-authorised trastuzumab reference product were compared by measuring PI and ADCC activity in HER2-overexpressing cell lines. PI activity was evaluated using BT-474 cells; ADCC was assessed using HCC2218 as target cells. Antitumour activity was compared in two mouse xenograft models: a BT-474 breast tumour model and an NCI-N87 gastric tumour model.
- Hanes v, et al. EBCC 2016; Abstract 436 and poster.
- Hutterer K, et al. WCBP Symposium 2017; Abstract P-207-TH and poster.
In vivo analysis
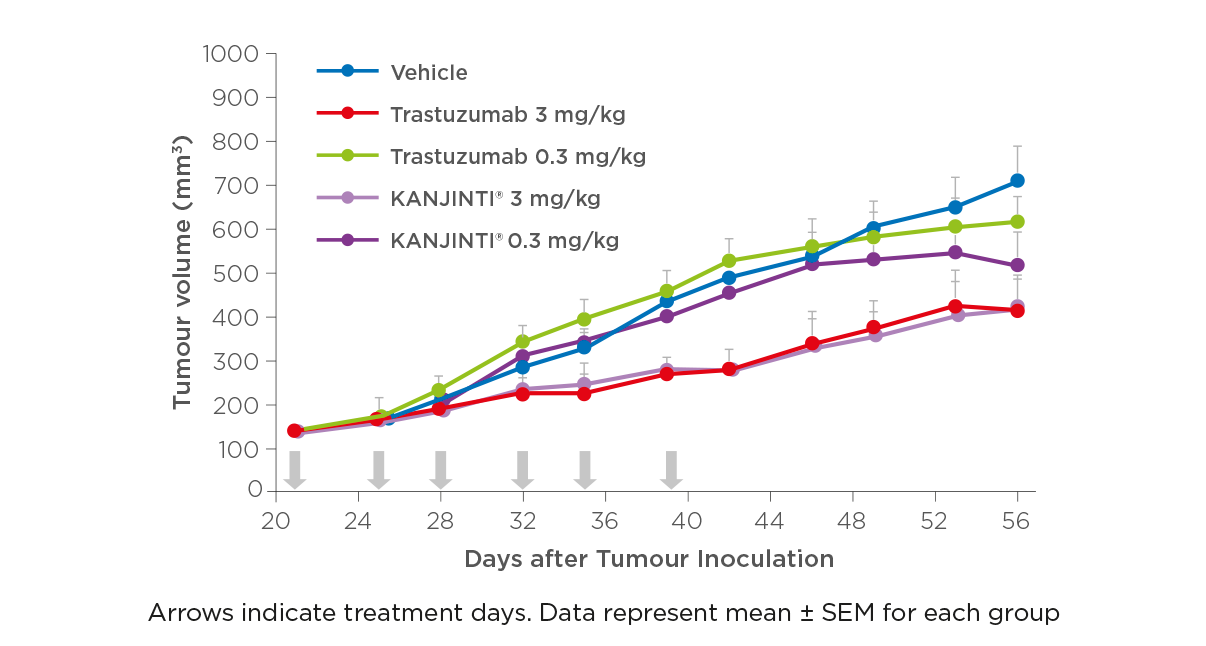
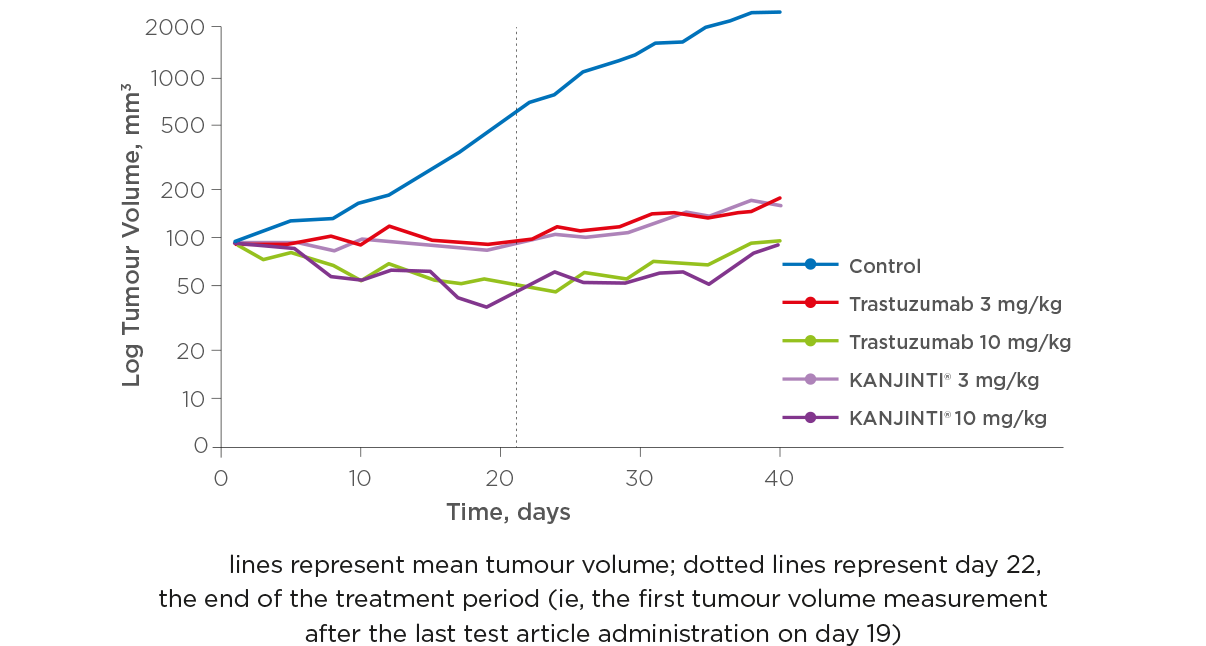
Different concentrations (3 mg/kg and 0.3 mg/kg) of KANJINTI® and the reference product were compared to understand the antitumour activity of each drug in two xenograft models, one for breast cancer and one for gastric cancer.
The results demonstrated that both KANJINTI® and the trastuzumab reference product had similar activity under the standard research conditions for these models.1
ANTITUMOUR ACTIVITY IN BREAST CANCER MODEL
ANTITUMOUR ACTIVITY IN GASTRIC CANCER MODEL
- Hanes v, et al. EBCC 2016; Abstract 436 and poster.
- Hanes v, et al. EBCC 2016; Abstract 436 and poster.
- Hutterer K, et al. WCBP Symposium 2017; Abstract P-207-TH and poster.


The information contained in this website is for European healthcare professionals only
I understand and confirm I am an EU healthcare professional
Read more >
Read more >
I am not an EU healthcare professional
Read more >
Read more >
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
