Countries ▼
KANJINTI®: A NEW CHAPTER IN AMGEN’S LEGACY OF BIOLOGIC DEVELOPMENT
KANJINTI® has been developed with the same rigour, with the same group of scientists and in the same manufacturing facilities as Amgen’s innovative biologics to ensure high quality.
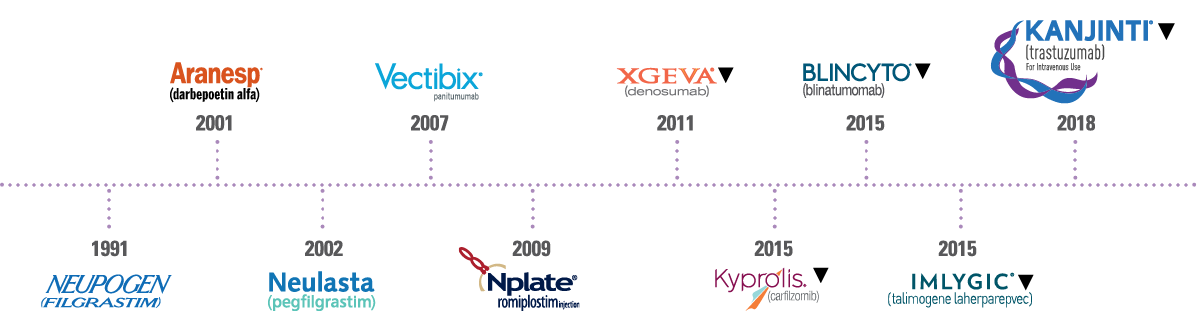
Amgen has been at the forefront of biologic innovation, particularly in oncology, and has served millions of cancer patients over the years. The reliability of Amgen’s manufacturing and supply provides confidence that its biologics are consistently delivered to serve every patient, every time.
For further information about Amgen’s ongoing commitment to delivering high quality and reliable products please follow these links:
Biologics expertise
Read more ►
Excellence in manufacturing
Read more ►
Reliability in product supply
Read more ►


The information contained in this website is for European healthcare professionals only
I understand and confirm I am an EU healthcare professional
Read more >
Read more >
I am not an EU healthcare professional
Read more >
Read more >
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
