Countries ▼
CLINICAL STUDY
LILAC TRIAL WAS A LARGE, RANDOMISED, DOUBLE-BLIND, PHASE 3 CLINICAL STUDY IN HER2+ EARLY BREAST CANCER PATIENTS
Study design
The trial was conducted in a sensitive patient population with sensitive endpoints. LILAC compared the efficacy and safety of KANJINTI® with Herceptin® (the trastuzumab reference product).1
THE ONLY CLINICAL STUDY FOR A TRASTUZUMAB BIOSIMILAR TO INCLUDE A SINGLE-SWITCH ARM
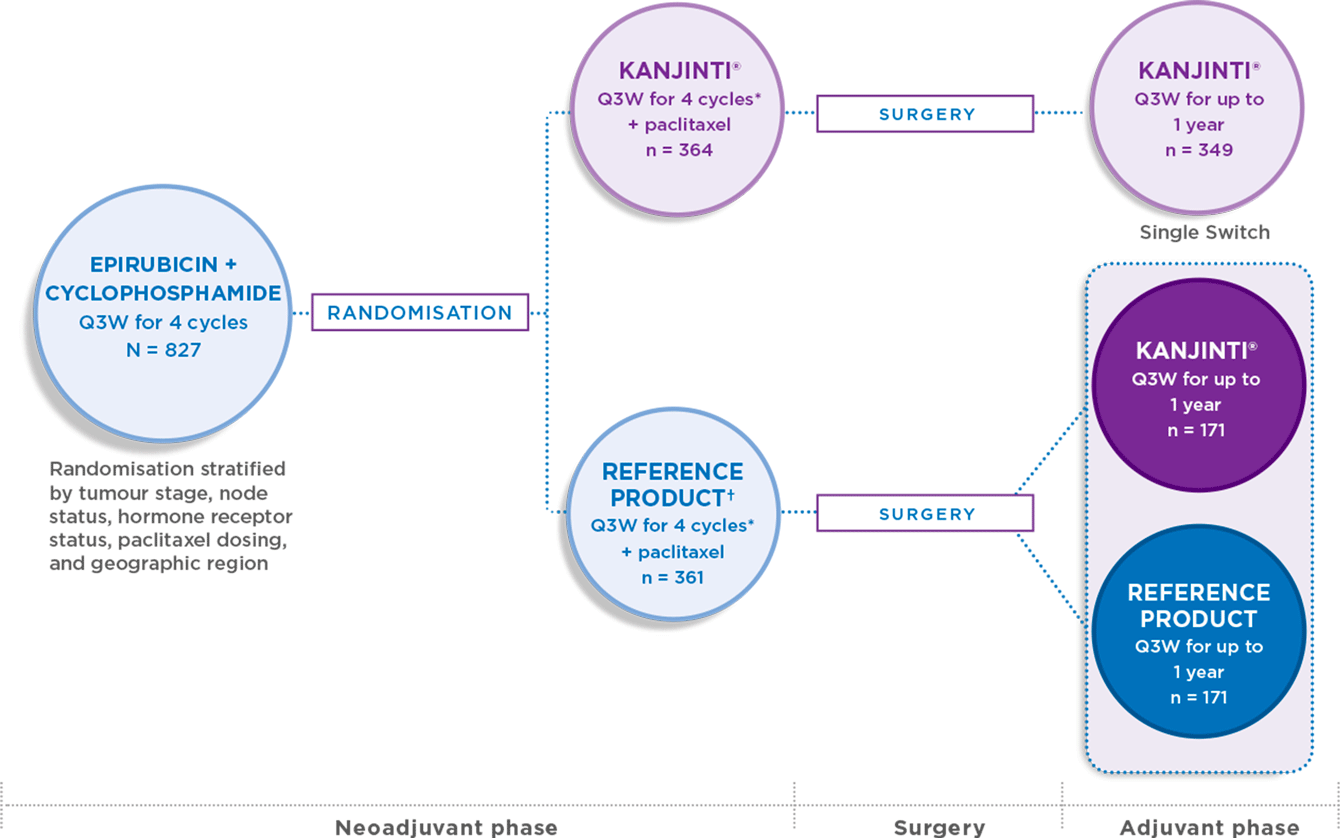
* Initial dose of 8 mg/kg IV then 6 mg/kg for remaining cycles. Paclitaxel could be administered weekly as per local protocols.
** Exclusion criteria: 1. Presence of bilateral breast cancer or known metastases; 2. Prior treatment including chemotherapy, biologic therapy, radiation or surgery forprimary breast cancer; 3. Concomitant active malignancy; 4. History of malignancy in the past 5 years, except treated basal cell carcinoma of the skin or carcinoma in situ of the cervix.
† Reference product is Herceptin®, a registered trademark of Roche, Germany.
** Exclusion criteria: 1. Presence of bilateral breast cancer or known metastases; 2. Prior treatment including chemotherapy, biologic therapy, radiation or surgery forprimary breast cancer; 3. Concomitant active malignancy; 4. History of malignancy in the past 5 years, except treated basal cell carcinoma of the skin or carcinoma in situ of the cervix.
† Reference product is Herceptin®, a registered trademark of Roche, Germany.
- HER2+ invasive breast cancer
- Histologically confirmed, measurable disease (≥ 2.0 cm)
- No prior treatment
- Planning for surgical resection of breast tumour and resection of sentinel node or axillary lymph node
- Planning neoadjuvant chemotherapy
- No distant metastases
- KANJINTI® was evaluated in both the neoadjuvant and adjuvant phases. Patients who received
KANJINTI® during the neoadjuvant phase continued to receive KANJINTI® Q3W for the adjuvant phase - Patients who received trastuzumab during the neoadjuvant phase were either switched to KANJINTI® Q3W or continued receiving trastuzumab for the adjuvant phase1
Clinical opinion concurs that the neoadjuvant setting offers a homogeneous population for detecting slight differences in safety and efficacy between a biosimilar and its reference product, should any differences exist.1
Efficacy results
In both new and switch patients, KANJINTI® was proven to have similar efficacy to the trastuzumab reference product.2
CO-PRIMARY ANALYSIS AND RIGOUROUS SENSITIVITY ANALYSIS DEMONSTRATED EQUIVALENT PATHOLOGICAL COMPLETE RESPONSE (pCR; PRIMARY ENDPOINT)1
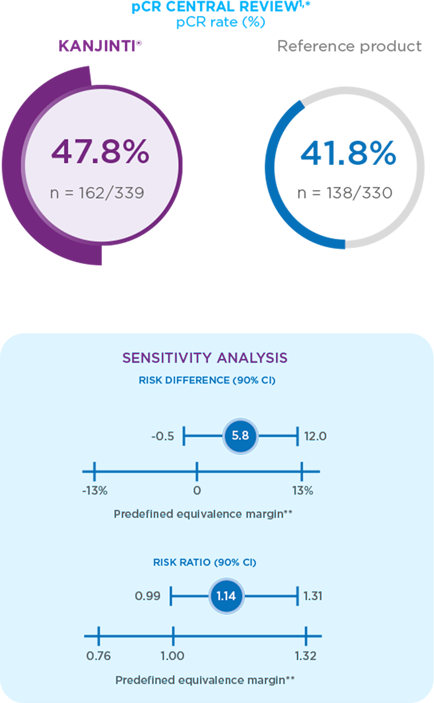
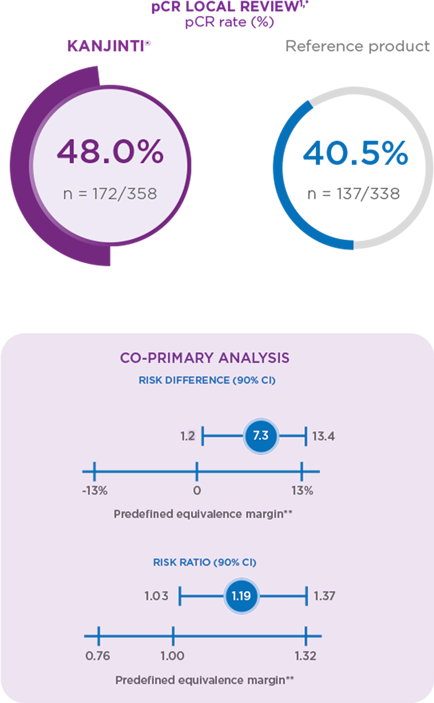
* pCR is defined as the absence of invasive tumour cells in the breast tissue and in axillary lymph nodes, regardless of ductal carcinoma in sit
(DCIS). Point estimate, confidence interval, and P value estimated using a generalised linear model adjusted for randomisation stratification
factors, tumour stage, node status, hormone receptor status, planned paclitaxel dosing schedule, and geographical region.
** In agreement with the European Medicines Agency.
** In agreement with the European Medicines Agency.
Safety and tolerability results
The LILAC study showed that the frequency, type, and severity of adverse events were similar between KANJINTI® and the trastuzumab reference product.1-3

Furthermore, the study reported no proven differences in immunogenicity or frequency of adverse events for patients who switched from trastuzumab to KANJINTI® after surgery, compared to those who remained on the trastuzumab reference product.1
- von Minckwitz G, et al. Lancet Oncol. 2018; doi: 10.1016/S1470-2045(18)30241-9.
- von Minckwitz G, et al. ESMO 2017; Abstract 151 PD and poster.
- Kolberg H-C, et al. SABCS 2017; Abstract PD3-10.


The information contained in this website is for European healthcare professionals only
I understand and confirm I am an EU healthcare professional
Read more >
Read more >
I am not an EU healthcare professional
Read more >
Read more >
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
